In the quest for cleaner and safer drinking water, researchers and engineers have developed various water filtration methods throughout the years. From basic charcoal filters to more advanced techniques, each method aims to remove impurities and contaminants, providing us with access to fresh and potable water. However, with so many options available, it can be challenging to determine which filtration method is the most advanced and effective. In this article, we will explore some of the top contenders in the field of water filtration and examine their strengths and limitations.
Reverse Osmosis (RO) Filtration
How RO Filtration Works
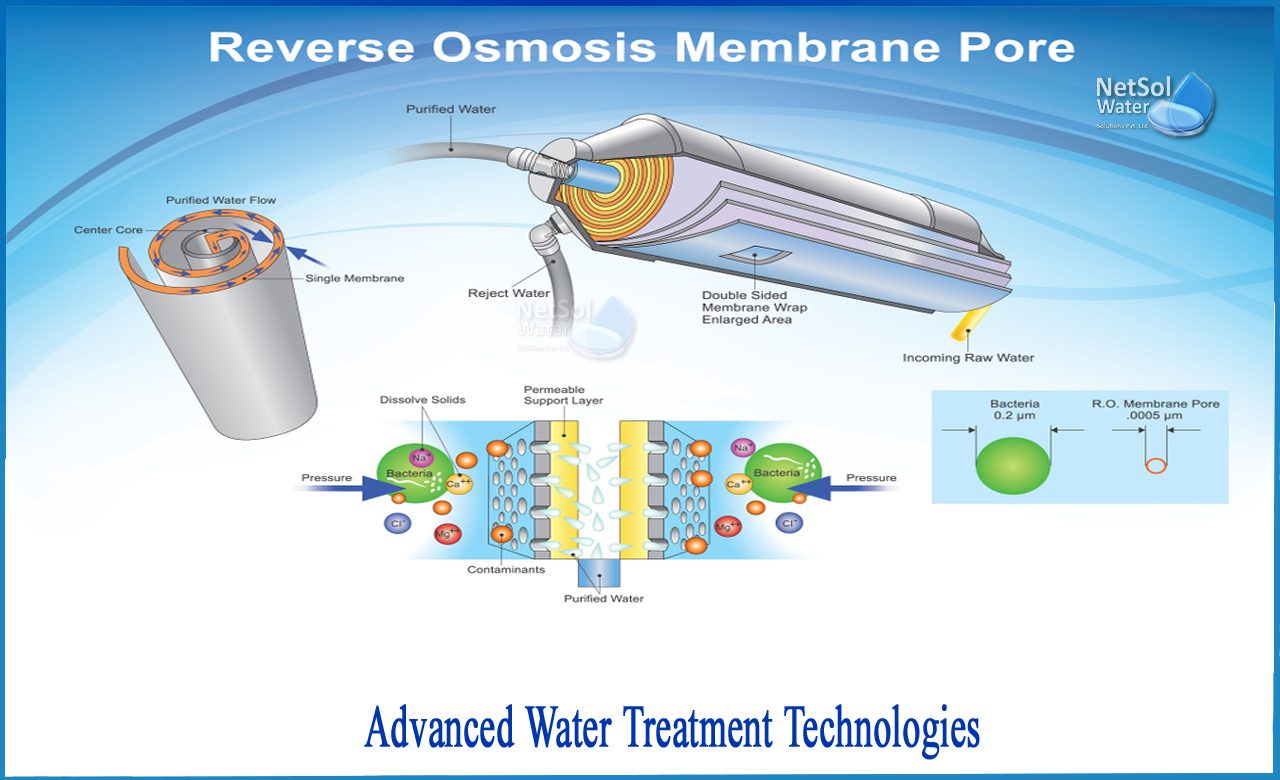
Reverse Osmosis (RO) Filtration is considered one of the most advanced water filtration methods available today. It works by using a semipermeable membrane to remove impurities, such as dissolved minerals, salts, and contaminants, from water. The process involves applying pressure to the water, forcing it through the membrane. The membrane only allows water molecules to pass through, while filtering out the impurities. The purified water then collects in a separate container, leaving behind the concentrated waste stream.
Pros and Cons of RO Filtration
There are several advantages to using RO Filtration. Firstly, it effectively removes a wide range of contaminants, including heavy metals, toxins, bacteria, and viruses, providing high-quality drinking water. Additionally, RO Filtration can improve the taste and odor of water by removing chlorine and other chemicals. It is also highly efficient in removing dissolved solids, making it ideal for locations with high levels of hardness or salinity in the water supply. Another benefit is that it does not require the use of chemicals, which makes it environmentally friendly.
However, there are also some disadvantages to consider. One of the main downsides is that RO Filtration can be quite slow, especially when compared to other filtration methods. The process requires multiple stages and can demand a significant amount of water pressure, resulting in a slower filtration rate. Another drawback is that RO Filtration tends to waste a considerable amount of water during the purification process. For every gallon of purified water produced, several gallons of wastewater are generated. This can be a concern in areas with water scarcity or high water costs.
Applications of RO Filtration
RO Filtration has a wide range of applications due to its effectiveness in removing contaminants. It is commonly used in residential settings, where it can provide purified drinking water for households. RO Filtration systems are also utilized in commercial and industrial settings, such as restaurants, hospitals, and manufacturing plants, where high-quality water is essential for various processes. Additionally, RO Filtration is frequently employed in desalination plants to convert seawater into freshwater. This technology plays a crucial role in providing drinkable water in areas facing water scarcity or relying on alternative water sources.
Activated Carbon Filtration
How Activated Carbon Filtration Works
Activated Carbon Filtration is another advanced water filtration method that utilizes activated carbon to remove impurities from water. Activated carbon has a porous structure, which increases its surface area and makes it highly effective in adsorbing contaminants. When water passes through an activated carbon filter, the carbon adsorbs organic compounds, chemicals, and some heavy metals, reducing their presence in the filtered water.
Pros and Cons of Activated Carbon Filtration
One of the significant advantages of Activated Carbon Filtration is its effectiveness in removing a wide range of organic compounds, chlorine, and volatile organic compounds (VOCs) that can affect the taste, odor, and quality of water. Additionally, the filtration process does not require electricity or complex infrastructure, making it a cost-effective solution for many applications. Activated Carbon Filtration also does not generate wastewater or produce harmful by-products, making it an environmentally friendly option.
However, there are limitations to consider. Activated Carbon Filtration is not as effective in removing inorganic compounds, such as salts, minerals, and heavy metals, which may require additional treatment methods. The efficiency of the filtration depends on the contact time between the water and the activated carbon, meaning that flow rates need to be adequately managed to ensure optimal performance. Another consideration is that the activated carbon needs periodic replacement to maintain its effectiveness, adding to the maintenance costs.
Applications of Activated Carbon Filtration
Activated Carbon Filtration is widely used in household water filters, improving the taste and quality of drinking water. It is also employed in point-of-use filters, shower filters, and refrigerator filters to remove chlorine and other impurities. In addition to residential applications, Activated Carbon Filtration finds extensive use in industrial settings. It is utilized in water treatment plants, food and beverage manufacturing, and laboratories to remove organic contaminants and improve the overall water quality. Furthermore, Activated Carbon Filtration is commonly integrated into air filtration systems to remove volatile organic compounds and odors.
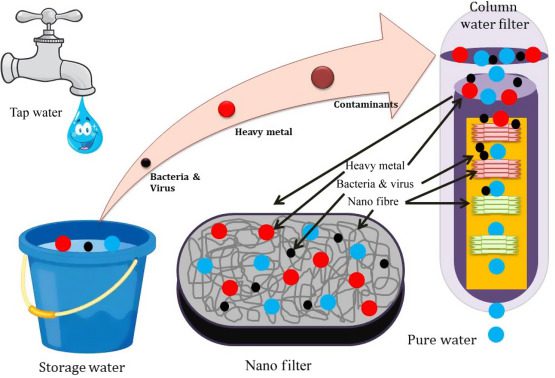

This image is property of ars.els-cdn.com.
Ultrafiltration
How Ultrafiltration Works
Ultrafiltration is a water filtration method that employs a thin membrane with small pores to separate water from dissolved substances and particles. The membrane used in ultrafiltration has a smaller pore size compared to microfiltration but larger than nanofiltration and reverse osmosis. The pressure applied to the water forces it through the membrane, leaving behind contaminants such as bacteria, viruses, colloids, and organic macromolecules.
Pros and Cons of Ultrafiltration
Ultrafiltration offers several advantages in water filtration. It effectively removes bacteria and viruses, making the water safer for consumption. The process also removes suspended particles, such as rust, sediment, and turbidity, improving the overall clarity of water. Another benefit is that ultrafiltration does not require chemical additives, ensuring that the filtered water remains free from chemical residues. Additionally, ultrafiltration does not remove beneficial minerals, which can be important for the taste and nutritional value of water.
However, there are some limitations to consider. Ultrafiltration does not effectively remove dissolved salts, minerals, or smaller molecules, which may require additional treatment methods, depending on the desired water quality. The effectiveness of ultrafiltration depends on the regular cleaning and maintenance of the membrane, as fouling can occur over time, reducing its filtration efficiency. The initial cost of setting up an ultrafiltration system can also be higher compared to other filtration methods.
Applications of Ultrafiltration
Ultrafiltration has various applications due to its ability to remove bacteria, viruses, and suspended particles. It is commonly used in water treatment plants to provide clean drinking water to households. Ultrafiltration systems are also employed in the food and beverage industry to remove contaminants and ensure product safety. Furthermore, ultrafiltration finds application in wastewater treatment, where it helps in removing pollutants and achieving higher water quality standards. Overall, ultrafiltration is a versatile filtration method suitable for various residential, commercial, and industrial applications.
Nanofiltration
How Nanofiltration Works
Nanofiltration is a water filtration method that utilizes a semipermeable membrane with smaller pores compared to ultrafiltration but larger than reverse osmosis. When water passes through the membrane under pressure, it selectively removes divalent ions, organic molecules, and small particles, while allowing monovalent ions, water molecules, and some dissolved compounds to pass through.
Pros and Cons of Nanofiltration
Nanofiltration offers several advantages in water filtration. It can effectively remove divalent ions, such as calcium and magnesium, which are responsible for water hardness. This helps in improving the overall taste, clarity, and softness of water. Nanofiltration is also efficient in removing organic molecules, bacteria, and viruses, making it a reliable method for producing safe drinking water. Additionally, it requires less pressure compared to reverse osmosis, resulting in lower energy consumption.
However, there are limitations to consider. Nanofiltration is less effective in removing monovalent ions, such as sodium and chloride, which may require additional treatment methods, depending on the desired water quality. The increased selectivity of nanofiltration comes at the cost of a lower flow rate, meaning that it may require larger filtration systems to meet higher throughput demands. Another consideration is the potential for membrane fouling, which can reduce the efficiency of the filtration process and necessitate regular maintenance.
Applications of Nanofiltration
Nanofiltration finds various applications in water treatment due to its selective removal capabilities. It is commonly used in treating groundwater and surface water sources, removing hardness-causing ions and improving water quality. Nanofiltration is also employed in the production of soft drinks and juices to remove impurities and enhance taste. Additionally, it is utilized in wastewater treatment and recycling processes to remove contaminants and produce water suitable for reuse. Nanofiltration has also found application in the pharmaceutical industry for the purification of process water and the removal of organic impurities.
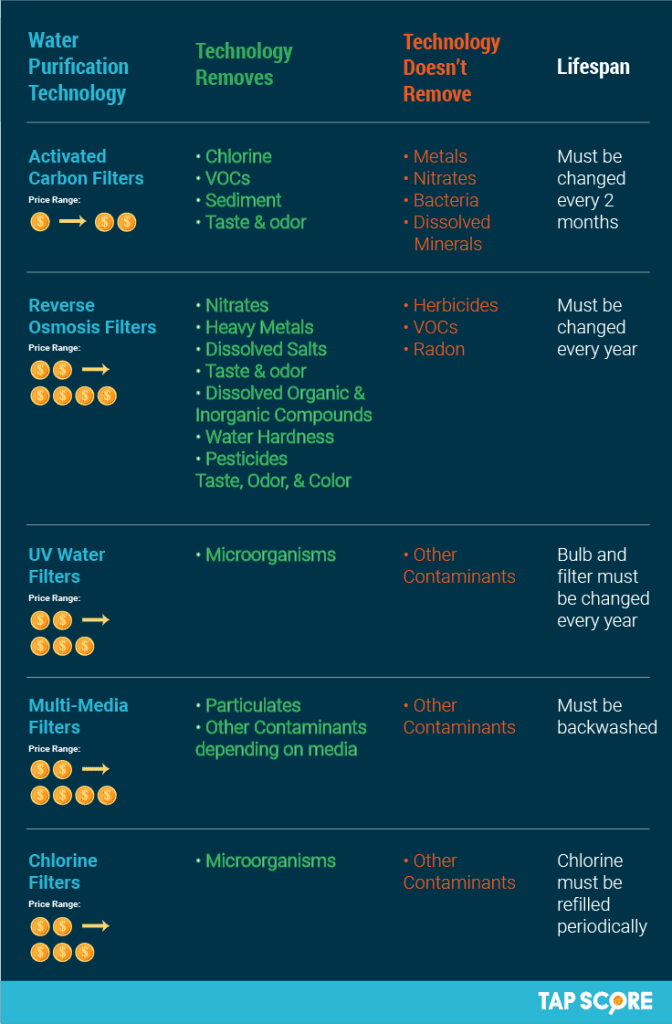

This image is property of cdn.shopify.com.
Ultra Violet (UV) Disinfection
How UV Disinfection Works
Ultra Violet (UV) Disinfection is a water treatment method that utilizes ultraviolet light to destroy or inactivate microorganisms, including bacteria, viruses, and parasites. The water is exposed to UV light, which damages the DNA or RNA of the organisms, preventing their ability to replicate and causing their death or inactivation.
Pros and Cons of UV Disinfection
UV Disinfection offers several advantages in water treatment. It is highly effective in killing a wide range of microorganisms, including bacteria, viruses, and parasites, without the need for chemicals. UV disinfection does not alter the taste, odor, or color of the water, making it suitable for applications where water quality is crucial. Additionally, it does not introduce any harmful by-products during the disinfection process, ensuring the safety and purity of the treated water.
However, there are limitations to consider. UV disinfection is reliant on the contact time between the UV light and the water to achieve sufficient disinfection. Therefore, proper flow rates and exposure time need to be maintained to ensure effective treatment. UV disinfection does not remove other impurities or contaminants from the water, such as chemicals or heavy metals, which may require additional treatment methods. Lastly, UV disinfection is dependent on a consistent and reliable power source, making a backup system essential to ensure continuous operation.
Applications of UV Disinfection
UV Disinfection is commonly used in residential, commercial, and industrial settings for water treatment and disinfection. It is frequently integrated into point-of-use filters, whole-house systems, and water storage tanks to provide safe drinking water. UV disinfection is also employed in swimming pools and spas to maintain proper water hygiene and prevent the spread of waterborne illnesses. Additionally, UV disinfection finds application in the food and beverage industry, healthcare facilities, and wastewater treatment plants to ensure the safety and purity of water used for various processes.
Electrodialysis
How Electrodialysis Works
Electrodialysis is a water filtration method that utilizes an electric field and selective ion exchange membranes to separate ions from water. The process involves passing water through alternating cation and anion exchange membranes, creating an electric potential that drives the movement of ions across the membranes. As a result, ions of opposite charges are selectively removed, leaving behind purified water.
Pros and Cons of Electrodialysis
Electrodialysis offers several advantages in water filtration. It effectively removes dissolved ions, such as salts, minerals, and heavy metals, making it suitable for desalination and the treatment of brackish water. Electrodialysis operates without the need for chemical additives, ensuring that the purified water remains free from chemical residues. Additionally, it requires fewer moving parts and has lower energy consumption compared to other filtration methods.
However, there are limitations to consider. Electrodialysis is dependent on the water’s ionic composition, meaning that the efficiency of the process may vary based on the water source. The process requires a stable and reliable power source to maintain the electric field, making a backup system essential for continuous operation. Another consideration is the potential for membrane fouling, which can reduce the effectiveness of the filtration process and necessitate regular cleaning and maintenance.
Applications of Electrodialysis
Electrodialysis finds various applications in water treatment and desalination processes. It is commonly used in desalination plants to remove salts and minerals from seawater, providing a source of freshwater. Electrodialysis is also employed in the treatment of brackish water, removing contaminants and improving water quality for various applications. Additionally, it finds application in the food and beverage industry, where it can be used to adjust the mineral content and improve the taste of water used for processes such as brewing or bottling.

This image is property of quenchwater.com.
Membrane Bioreactor (MBR) Filtration
How MBR Filtration Works
Membrane Bioreactor (MBR) Filtration combines the principles of biological treatment and membrane filtration to remove contaminants from water. It involves the use of a bioreactor, where microorganisms break down organic compounds, and a membrane filtration system, which separates the treated water from the suspended solids and microorganisms present in the bioreactor. This results in high-quality, treated water that meets stringent water quality standards.
Pros and Cons of MBR Filtration
MBR Filtration offers several advantages in water treatment. It provides excellent effluent quality, removing suspended solids, microorganisms, and organic compounds effectively. The process is highly reliable and can handle fluctuations in influent water quality and quantity. MBR Filtration also requires a smaller footprint compared to conventional treatment processes, making it suitable for locations with limited space. Another benefit is that the treated water from an MBR system can be reused for various applications, reducing water wastage.
However, there are limitations to consider. MBR Filtration requires a significant initial investment and can have higher operating costs compared to conventional treatment methods. The membranes used in MBR systems are prone to fouling, necessitating regular cleaning and maintenance. Additionally, MBR Filtration may require backup systems or redundancy to ensure continuous operation, as any mechanical or electrical failures can disrupt the treatment process.
Applications of MBR Filtration
MBR Filtration finds various applications in water treatment and wastewater treatment processes. It is commonly used in municipal water treatment plants to achieve stringent effluent standards and produce high-quality water. MBR systems are also employed in industrial settings, such as food and beverage manufacturing, pharmaceutical production, and chemical processing, where reliable and efficient treatment is required. Furthermore, MBR Filtration is utilized in decentralized wastewater treatment systems, serving small communities, remote areas, or buildings with limited access to public sewer systems.
Ion Exchange
How Ion Exchange Works
Ion Exchange is a water filtration method that involves the exchange of ions between a solid resin material and the water being treated. The resin material contains charged ions capable of attracting and replacing ions of the same charge present in the water. This process effectively removes dissolved ions and replaces them with different ions, resulting in the desired water quality.
Pros and Cons of Ion Exchange
Ion Exchange offers several advantages in water filtration. It can effectively remove dissolved salts, minerals, heavy metals, and other contaminants, making it suitable for water softening, demineralization, and purification. Ion Exchange can be customized to target specific ions or contaminants, allowing for precise control over the water quality. Additionally, the resin material used in Ion Exchange can be regenerated, reducing the need for continuous replacement and making it a cost-effective solution.
However, there are limitations to consider. Ion Exchange is not effective in removing organic compounds, bacteria, or viruses from water, which may require additional treatment methods. The process also requires careful monitoring and maintenance to ensure optimal resin performance and prevent fouling or exhaustion. Another consideration is the disposal and regeneration of spent resin, which may have environmental implications.
Applications of Ion Exchange
Ion Exchange is widely used in various water treatment applications. It is commonly utilized for water softening, where it removes hardness-causing ions, such as calcium and magnesium, and replaces them with sodium or hydrogen ions. Ion Exchange is also employed in the demineralization of water, removing salts and minerals to achieve a desired water quality. Additionally, it finds application in industrial processes where the removal of specific ions or contaminants is necessary, such as in the production of high-purity water for electronics manufacturing or pharmaceutical production.
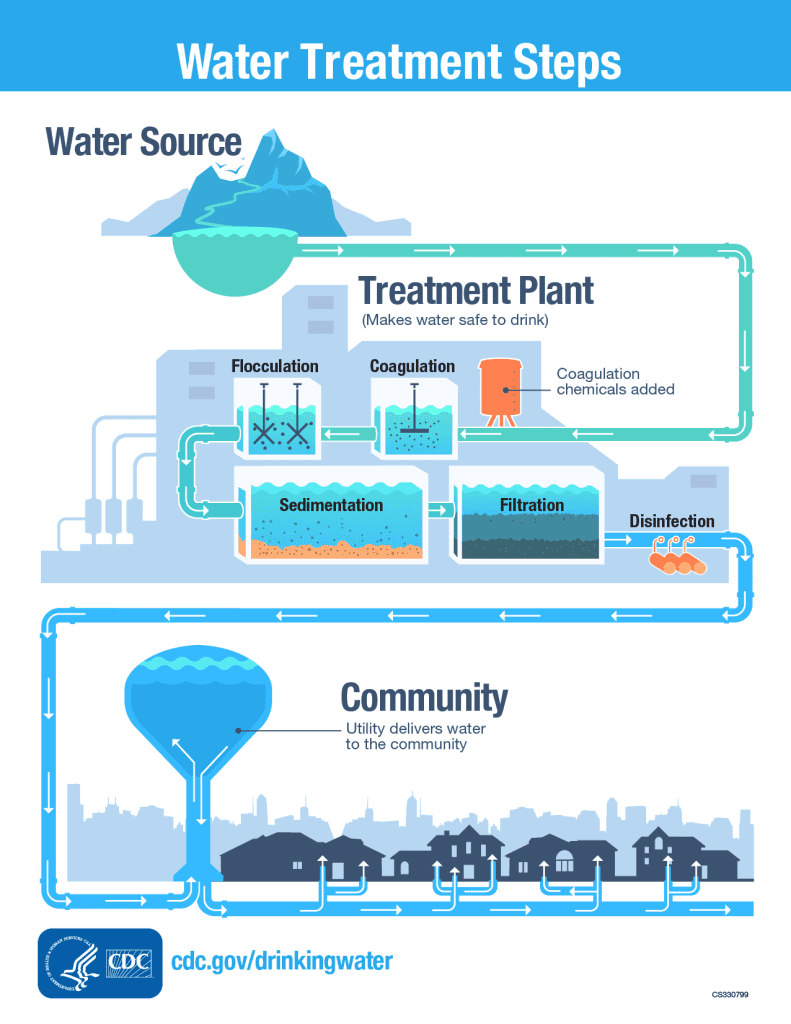

This image is property of www.cdc.gov.
Electrodeionization (EDI)
How Electrodeionization Works
Electrodeionization (EDI) is a water purification method that combines ion exchange and electrodialysis principles. It involves the use of ion exchange membranes and an electric field to remove ions from water. The process relies on the movement of ions between ion exchange resins and electrode compartments, resulting in the continuous removal of ions, including dissolved salts and other contaminants.
Pros and Cons of Electrodeionization
Electrodeionization offers several advantages in water purification. It provides a continuous and chemical-free method of removing ions from water, resulting in high-purity water suitable for various applications. Electrodeionization systems generally require less maintenance compared to traditional ion exchange systems, as they do not require the regeneration of resins or the disposal of spent chemicals. Another benefit is that EDI systems have lower energy consumption compared to other water purification methods, making them environmentally friendly and cost-effective.
However, there are limitations to consider. Electrodeionization is not effective in removing organic compounds, bacteria, or viruses from water, which may require additional treatment methods. The efficiency of the process depends on the regular cleaning and maintenance of the ion exchange membranes, as fouling can occur over time and reduce performance. Additionally, the initial investment for setting up an EDI system can be higher compared to other purification methods.
Applications of Electrodeionization
Electrodeionization finds various applications in water treatment and purification processes. It is commonly used in the production of ultrapure water for industries such as semiconductors, power generation, and pharmaceuticals. EDI systems are also employed in the production of high-quality drinking water, where the removal of ions and contaminants is crucial for meeting water quality standards. Additionally, EDI finds application in the food and beverage industry, laboratory settings, and specialty chemical manufacturing.
Conclusion
The most advanced water filtration method depends on the specific requirements and desired water quality for each application. Reverse Osmosis (RO) Filtration is highly effective in removing a wide range of contaminants, making it suitable for residential, commercial, and industrial settings. Activated Carbon Filtration excels in removing organic compounds and chemicals, enhancing the taste and quality of drinking water. Ultrafiltration and Nanofiltration are reliable methods for removing bacteria, viruses, and suspended particles, with different pore sizes catering to varying filtration needs. Ultra Violet (UV) Disinfection offers a chemical-free solution for safely treating water, killing microorganisms without altering its taste or odor. Electrodialysis, Membrane Bioreactor (MBR) Filtration, Ion Exchange, and Electrodeionization provide efficient ways to remove various ions, salts, and contaminants from water, catering to different industrial and municipal applications. Ultimately, the choice of the most appropriate water filtration method depends on factors such as water source, desired water quality, application, and cost considerations.
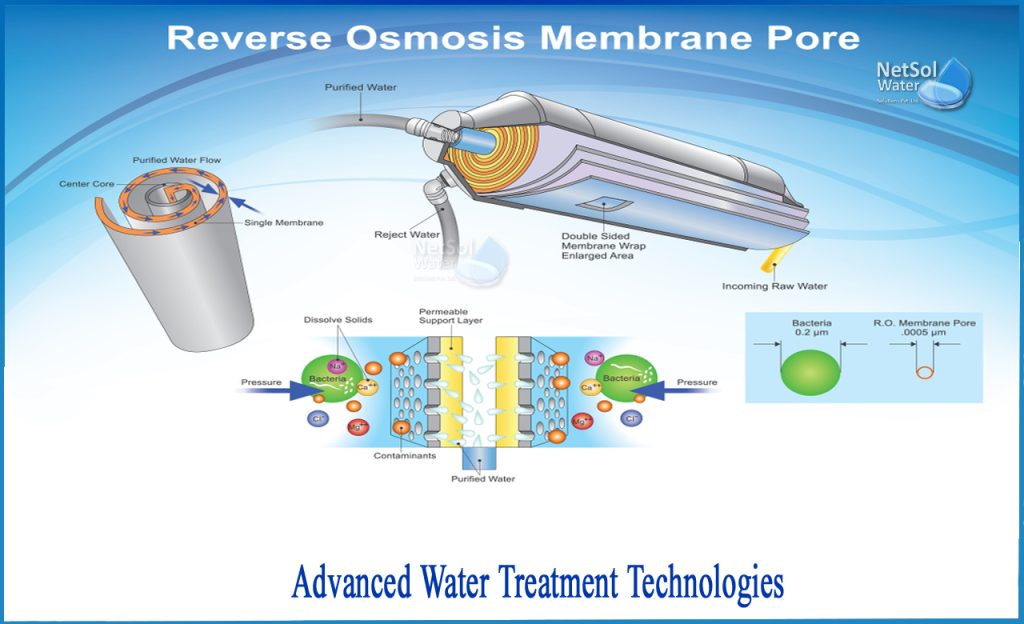

This image is property of www.netsolwater.com.